
What is Product Information Files, PIF?
Taiwan Food and Drug Administration (TFDA) announced the “Regulations for Cosmetic Product Information File Management" and the "Categories of Cosmetics Requiring Product Information Files and Implementation Dates" on May 30, 2019. According to this announcement, existing "Special Purpose Cosmetic" licenses valid until June 30, 2024, will be converted into "Product Information Files" (PIF).
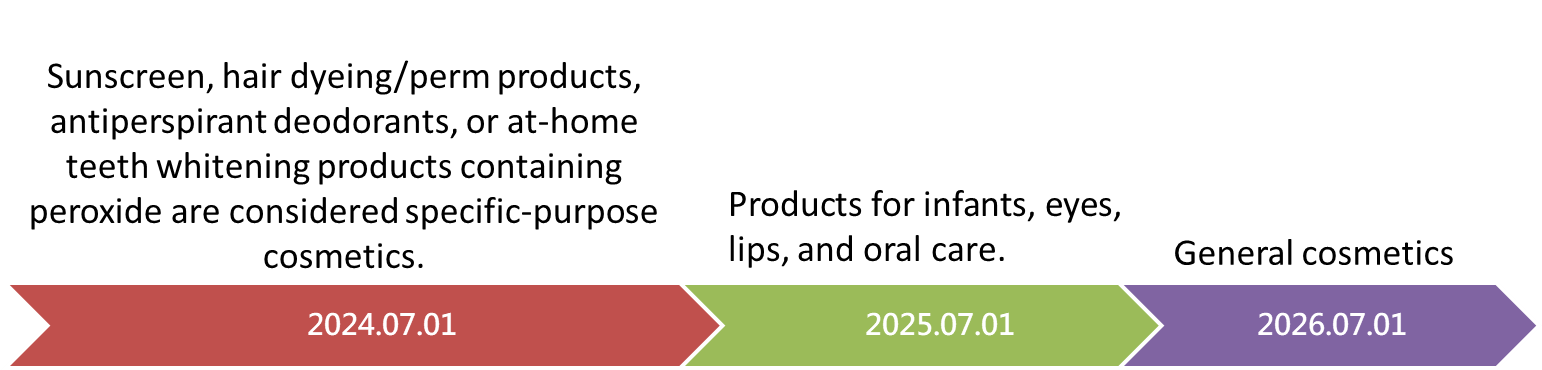
Starting from the effective date of the new announcement, cosmetic product registration will cease, and the implementation of Product Information File (PIF) establishment will be carried out in three phases according to product categories, as shown in the figure below. Regardless of whether the product is manufactured, imported, freebie, or for trial use, a "Product Information File" must be completed according to its product category. This announcement change represents a significant transformation for the cosmetics industry.
What is a "Product Information File" (PIF) for cosmetics?
Recently, new regulations and requirements for cosmetic product quality management have been introduced in various countries around the world. The PIF is designed to systematically collect related documents before a product is launched, including basic product information, GMP (Good Manufacturing Practices) of the manufacturer, packaging details, technical data, and safety assessment reports confirmed by qualified professionals. This ensures enhanced self-management by businesses, improved product quality, and consumer safety while enabling Taiwanese cosmetics to align more closely with international standards.
Who Can Serve as Safety Professionals?
According to the requirements for safety and efficacy evaluations in creating the PIF, qualified professionals must collect, verify, summary and sign off on information regarding product ingredients' characteristics, toxicology, absorption, metabolism, and adverse reactions. When submitting the data, these professionals must attach proof of their qualifications. If updates are made to the PIF, the product's safety must be reassessed.
The qualifications for safety professionals as following:
- Educational Background: Graduates from domestic or international programs in medicine, toxicology, or cosmetics-related fields.
- Till June 30, 2019, Graduates from domestic or international chemistry/chemical engineering programs with over five years of work experience in cosmetic safety assessments.
Additionally, the professionals must have certification from cosmetic safety assessment training courses to qualify for signing PIF safety data. The establishment of PIF also facilitates manufacturers for explore international markets. For instance, products intended for export to the EU or ASEAN countries can use the PIF for submission, reducing preparation time. PIF-related documents must be retained for five years after the product is launched. Any changes during this period require updates and re-approval by the safety professional.
Don’t know how to prepare Cosmetic Product Registration and PIF? Want to know how can establishing the PIF to meet requirements?
TALENT’s team has extensive experience in writing technical documents, product registration, conducting product safety assessments We also have qualified safety professionals who meet the requirements of TFDA to review and sign safety data.
If you need assistance, please don’t hesitate to contact us. We are here to support your compliance and ensure your products meet regulatory standards efficiently and professionally.
Reference:



