


TALENT provides services such as product registration, regulatory consulting, clinical trial management, post-marketing surveillance (PMS) study for pharmaceutical and medical device.

Provide high-quality and one-stop regulatory services
Meet the clients' needs of regulatory registration submitted in different countries

Rich practical experience
Professional seniority over 25 years
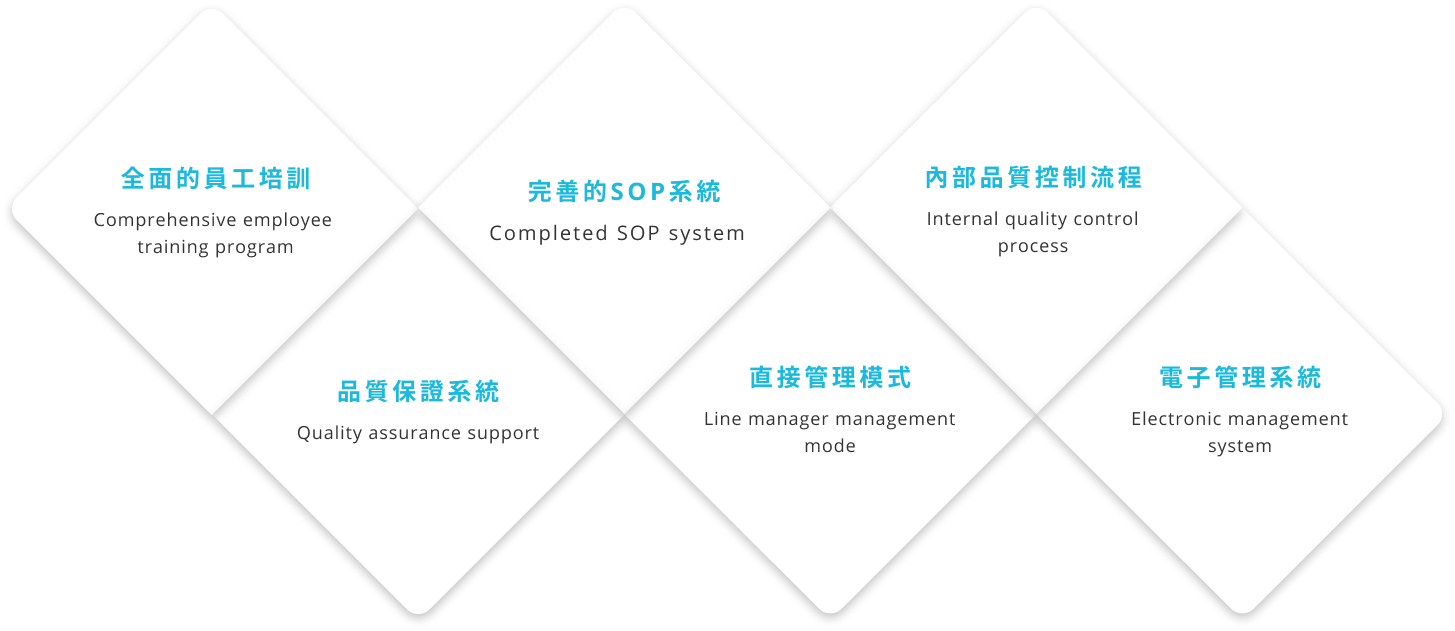
Talent provides high-quality and one-stop regulatory services, and can meet customers' needs for regulatory services submitted for review in different countries. Talent can act as the client's regulatory representative in Taiwan, directly helping clients communicate with regulatory agencies and submit documents for review.
Talent provides full service for clinical trials of drugs/medical equipment. Through its rich clinical trial experience, Talent provides clinical trial services that best meet the needs of customers in terms of price, quality and schedule. .
Our consulting services are as follows
● Product inspection and registration strategy
● Quality system pre-audit
● Quality system pre-audit
TALENT received permission for the Pharmaceutical Dealer License on November 23, 2023; providing our clients with a more comprehensive service. According to the Taiwan Pharmaceutical Affairs Act, a designated license holder in Taiwan is required for overseas manufacturers to apply for a pharmaceutical license from the Taiwan Food and Drug Administration, and now TALENT could be your designated license holder now.
The purpose of implementing monitoring during the clinical trial is to ensure quality operation and data as well as trial efficiency. Sponsors should develop a clinical trial monitoring plan based on the benefit and protection of the subjects, along with the accuracy and completeness of the data, to ensure that the trial is conducted under appropriate monitoring. The sponsor would appoint the Clinical Research Associate (CRA) and choose site monitoring, system remote monitoring, or both.
