


TALENT provides services such as product registration, regulatory consulting, clinical trial management, post-marketing surveillance (PMS) study for pharmaceutical and medical device.

Provide high-quality and one-stop regulatory services
Meet the clients' needs of regulatory registration submitted in different countries

Rich practical experience
Professional seniority over 25 years
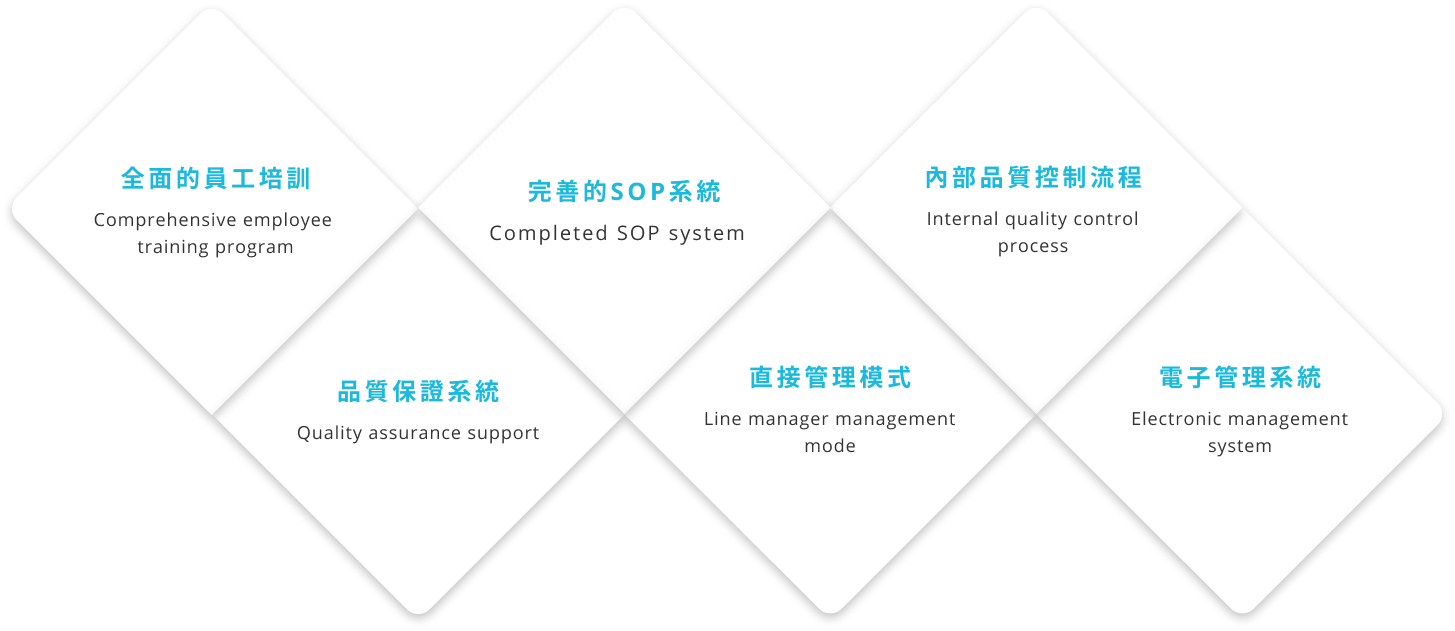
Talent provides high-quality and one-stop regulatory services, and can meet customers' needs for regulatory services submitted for review in different countries. Talent can act as the client's regulatory representative in Taiwan, directly helping clients communicate with regulatory agencies and submit documents for review.
Talent provides full service for clinical trials of drugs/medical equipment. Through its rich clinical trial experience, Talent provides clinical trial services that best meet the needs of customers in terms of price, quality and schedule. .
Our consulting services are as follows
● Product inspection and registration strategy
● Quality system pre-audit
● Quality system pre-audit
TALENT CRO Inc. will meet you at booth M1031!
Bio Asia-Taiwan 2025 will be held July 24-27, in Taipei, Taiwan. This event is the biggest bio exhibition in Asia and it brings thousands professional visitors to attending.
